Ennov: ライフサイエンス向けソフトウェアプラットフォーム
製品ライフサイクル全体をサポートし、強化するユニファイド・プラットフォーム

Gain unlimited access to our library of exclusive content

Ennovインサイダーになる
限定コンテンツのライブラリに無制限にアクセス
Ennovユーザーの成功事例をご覧ください。
Ennov
Ennovは、20年以上にわたり、文書およびプロセス管理のための革新的で強力かつ使いやすいソフトウェアを開発してきました。
Ennovのソリューションは、規制対象のコンテンツやプロセス管理に特化して設計されたコンプライアンスを対象としたユニファイド・プラットフォームをベースに構築されています。
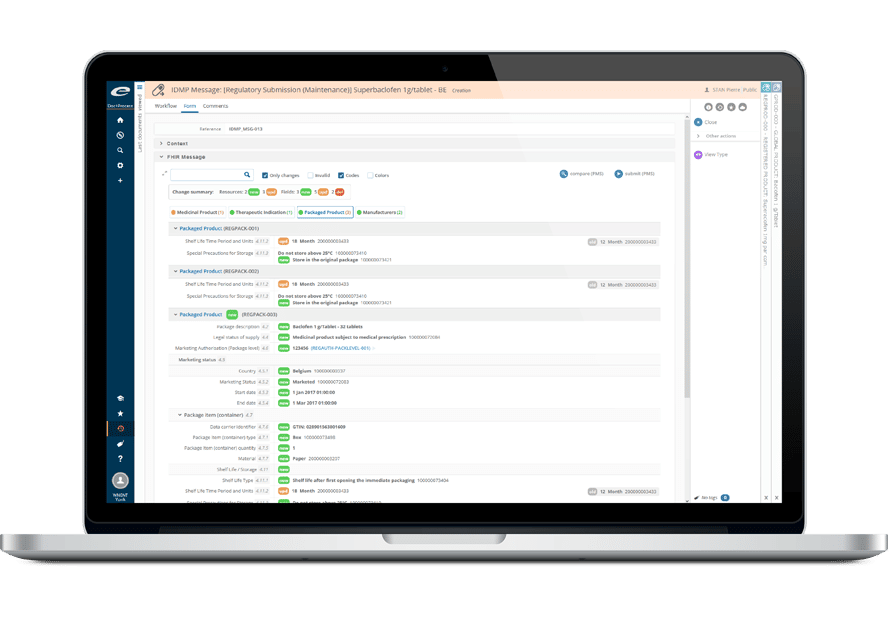
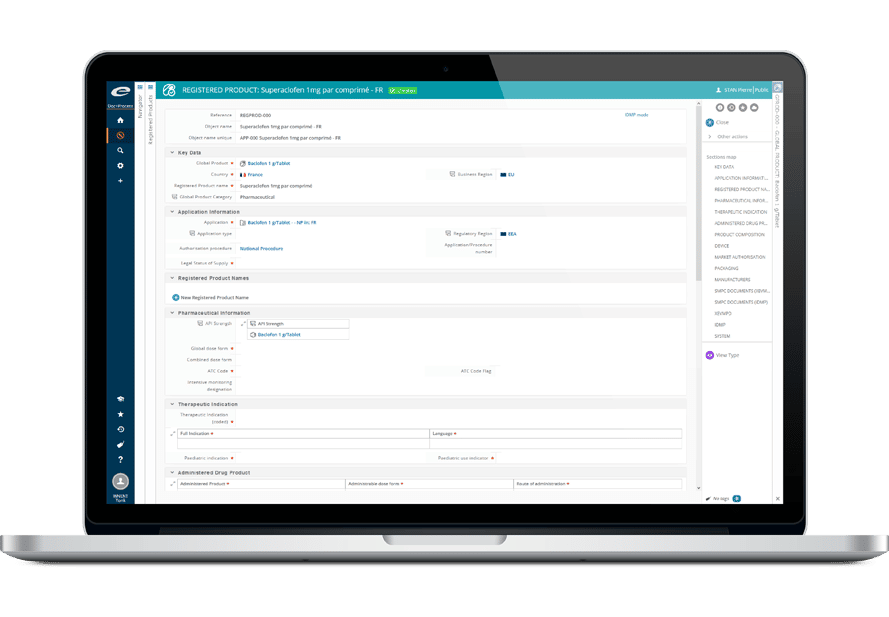
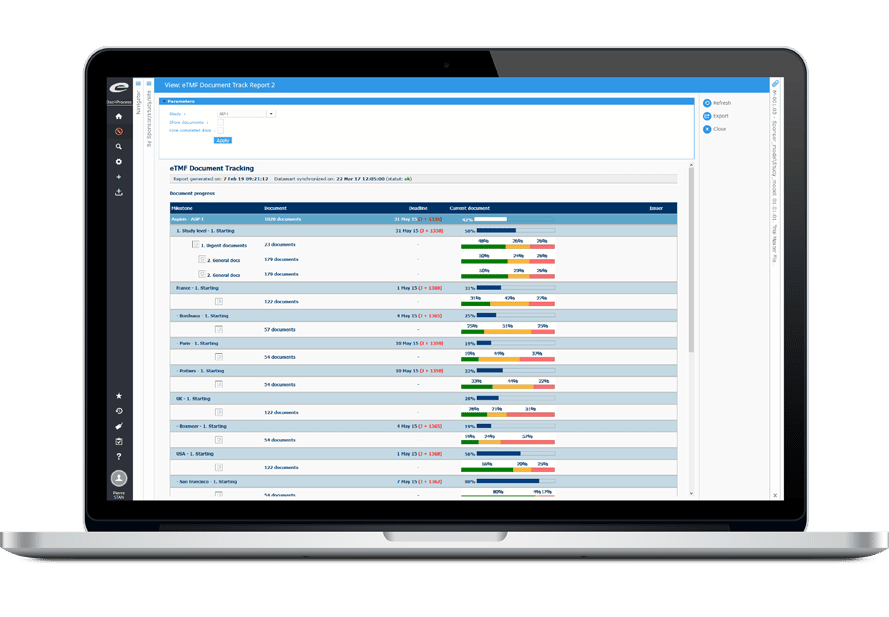
Ennovプラットフォームは、Regulatory(EDMS、Dossier Publishing、RIM、IDMP)、Quality(EDMS、QMS)、Clinical(eTMF、CTMS)の各ソリューションの技術的基盤となっています。
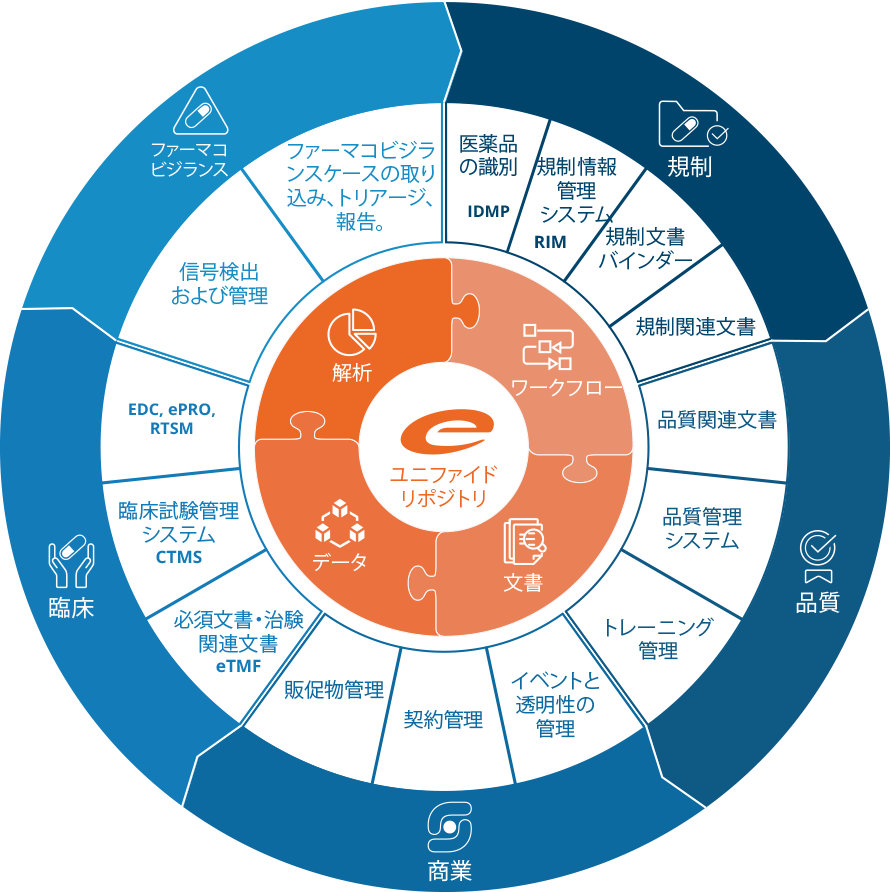
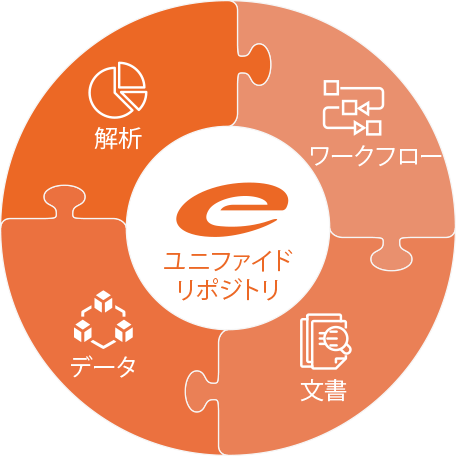
Ennov プラットフォーム
Ennov統一コンプライアンス・プラットフォームは、業務プロセス管理、文書管理、データ/フォーム管理、学習管理、ビジネス・インテリジェンスを組み合わせた高いレベルで設定可能なソフトウェア・アーキテクチャで、弊社アプリケーション構築の基盤となっています。
Ennov Regulatory
世界最高水準の規制コンテンツと情報管理
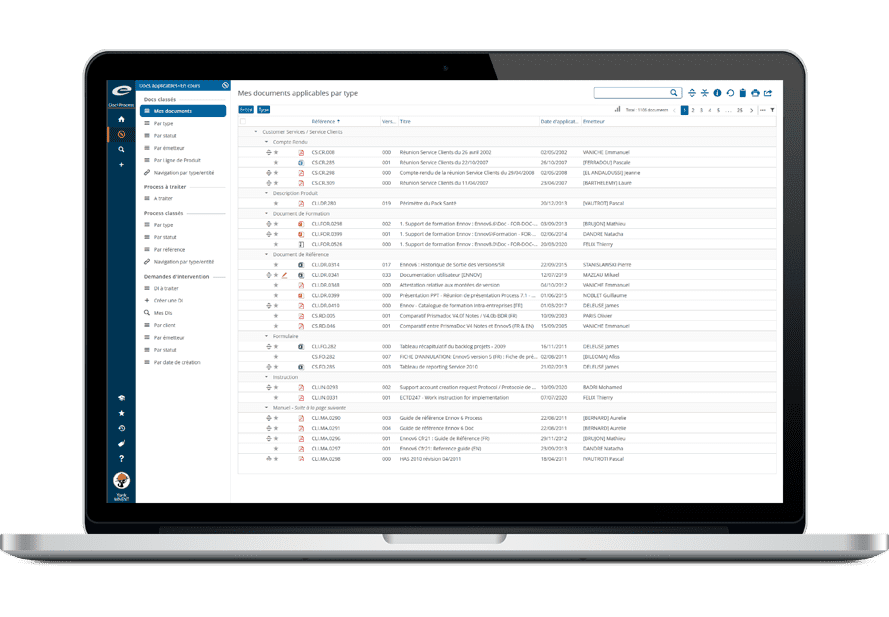
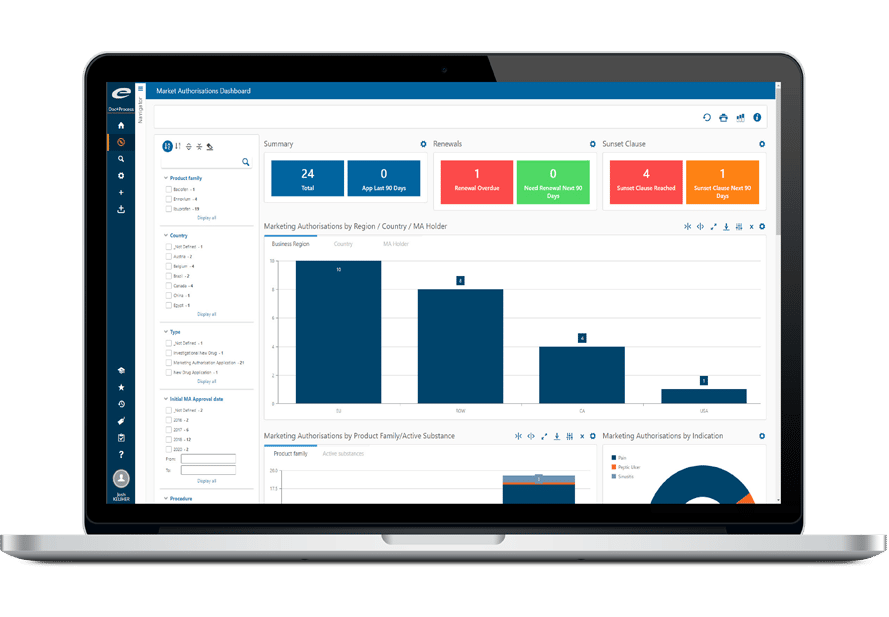
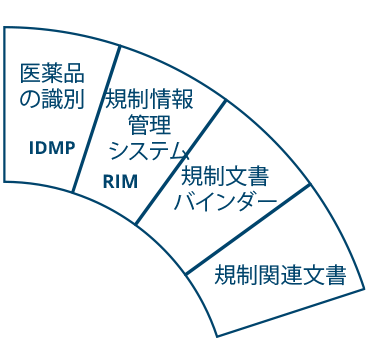
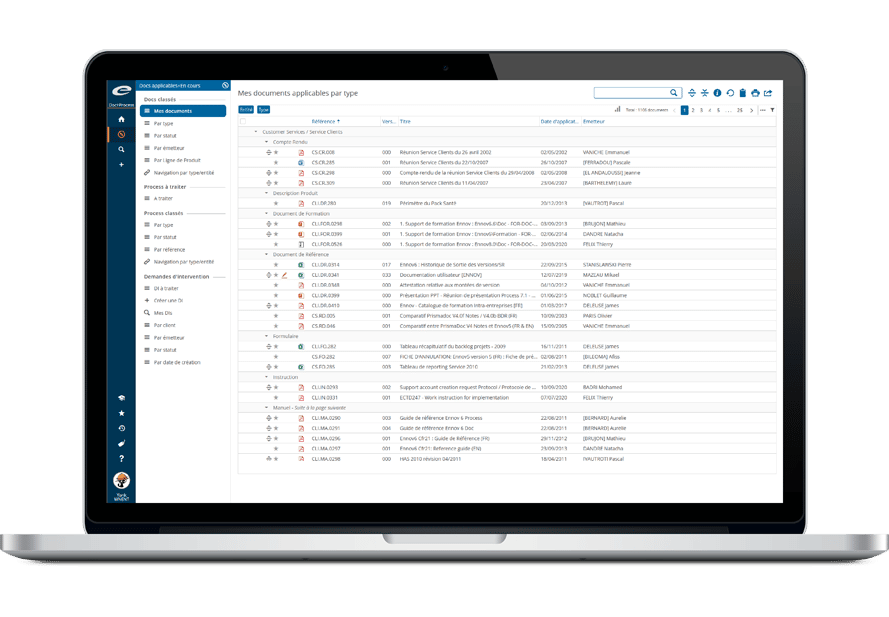
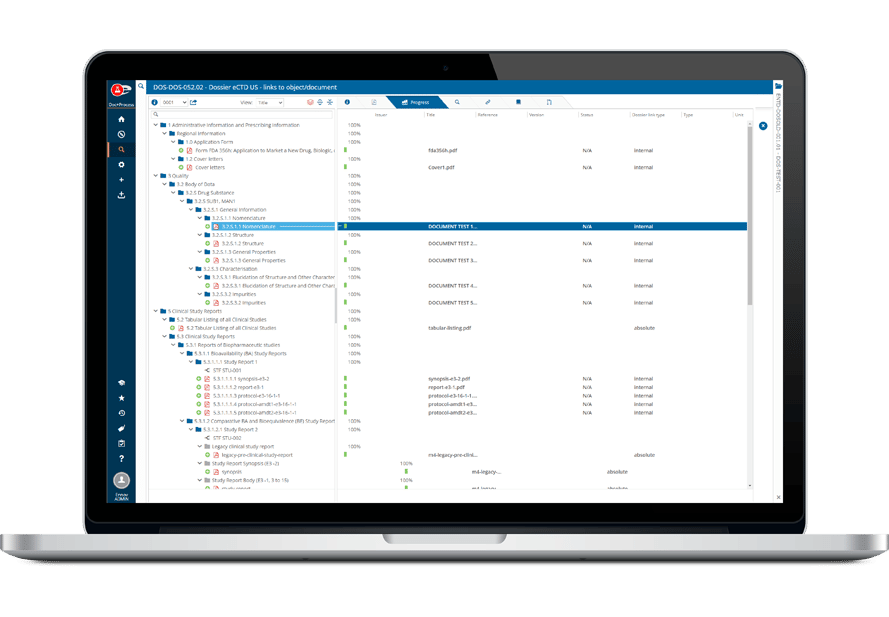
Ennov Regulatory Suite は Ennov Doc、Ennov Dossier、Ennov Process の機能と柔軟性を組み合わせ、登録目標の初期計画から製品の廃止まで、規制当局の製品ライフサイクル全体にわたってサポートするものです。Ennov Regulatory Suiteは、規制当局の活動計画、製品登録管理、資料作成、資料管理などにおいて非常に有用なツールです。
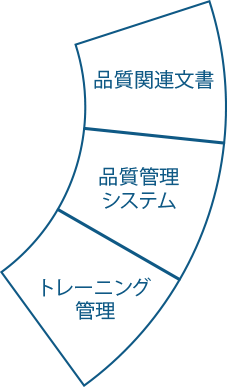
Ennov Quality
効率向上とコンプライアンス確保を実現する包括的なQMS
Ennov Qualityは、業界標準とベストプラクティスに基づいた品質文書、プロセス、ワークフローの定義済みインベントリを提供します。これにより、Ennov Qualityをご利用のお客様は、システムを迅速に本稼動させ、投資対効果を実感できるようになります。Ennov Qualityは、他のEnnovソリューションと同様、設定が簡単でITスキルを必要としません。
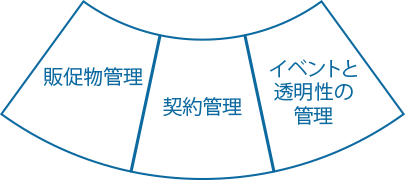
Ennov Commercial
イベント企画と経費の一元管理
Ennov Commercialは、会議、大会、シンポジウム、顧客コミュニティなどの職業イベントのあらゆる側面を計画、組織化、監視し、物流、予算、規制遵守を可視化、制御する包括的なソフトウェア・ソリューションです。
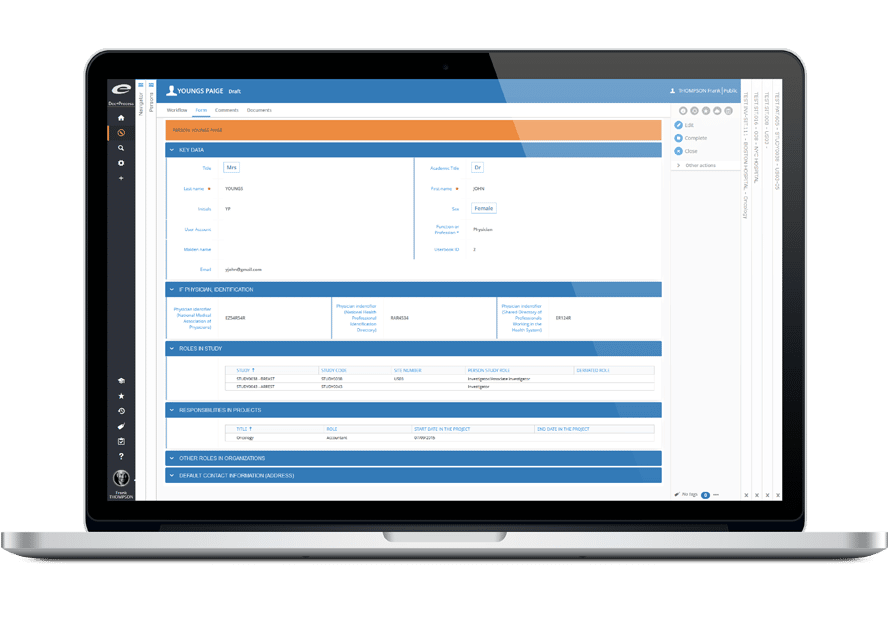
Ennov Clinical
臨床試験情報の取得と管理のための包括的な
ソリューション
Ennov Clinical Suiteは、臨床データ管理アプリケーション(EDC、RTSM、ePRO)と臨床試験管理アプリケーション(CTMS、eTMF)で構成されており、クラウドまたはオンプレミスで展開可能です。
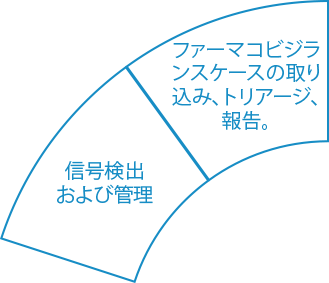
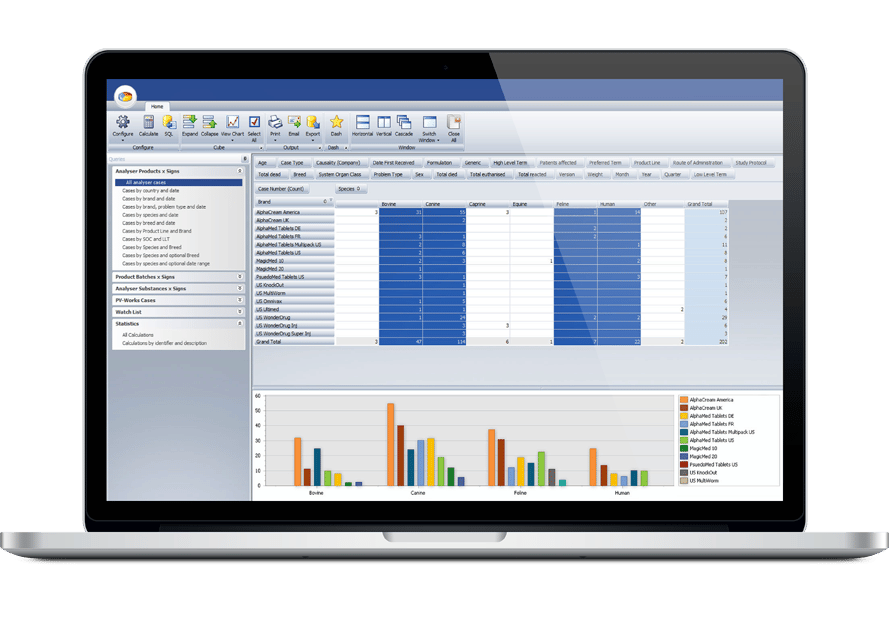
Ennov Pharmacovigilance
ヒトと動物のPVデータを収集、報告、分析するためのエンドツーエンド・ソリューション
Ennov Pharmacovigilance Suiteは、ヒトまたは動物の有害事象の収集、管理、評価、報告を1つの統一データベースに保持し、さらに高度なシグナル検出とPVデータ解析ツールを備えています。
Ennovコミュニティのメンバーになろう
世界250社以上のライフサイエンス企業が Ennovソリューションを導入



リソース
20年以上にわたる研究、イノベーション、開発から得られた知識とベストプラクティスを
、ヘルスおよびライフサイエンス分野でご活用ください。
ライフ向けの革新的ソフトウェア・ソリューション
結果重視のお客様本位のエクスペリエンスを提供
顧客満足度
20年以上にわたり、革新的で強力で使いやすいソフトウェアをライフサイエンス分野に提供してきた経験
専門スタッフ
平均顧客関係期間
顧客監査の成功率
お客様に充実したプロフェッショナル・サービスを提供

ホスティング
"弊社の柔軟な導入オプションは、お客様に選択の自由を提供"

実装
"最適化されたデリバリ方法論で、ソフトウェアの実装を迅速化"
トレーニング
"対象を絞った知識
移転による顧客の
成功を保証"

サポート
"ヘルプが必要な際、電話または
クリックするだけ"

コミュニティ
"すべてのお客様の
利益のためにお客様に寄り添う"
世界規模でのサービスとサポート
世界中で25万人以上のユーザーにサービスを提供していることが誇りです
始めましょう
どのようなご用件でしょうか?
下のフォームにご記入いただければ、すぐに連絡いたします
LinkedInで Ennovをフォローしてください
linkedin.com/company/ennov
Facebookで Ennovをフォローしてください
facebook.com/EnnovJP