Ennov regulatory suite
Ennov Artwork
アートワーク管理ソフトウェア
- ミスを減らすことで、市場投入までの時間を短縮
- アートワーク・ファイルの一元管理
- 高度なバージョニングと注釈ツール
- 他のEnnovコアモデルとシームレスに統合
- 100%ウェブベースで操作が簡単
ライフサイエンス業界におけるアートワーク・コンプライアンスの課題
ライフサイエンス業界において、包装資材に印刷されているアートワークの管理には多くの課題があります。
企業は、厳しいスケジュールとプロジェクト管理のプレッシャーに直面する一方で、関係者間で効率的に協力する必要が生じています。
承認ワークフロー、リソース管理、フィードバックループなどが複雑なため、法令順守を徹底するとともに、ミスを最小限に抑えることが重要です。
断片化したアートワーク・ファイルやバージョンの取り扱いは、混乱、頻繁なミス、市場投入の遅延につながります。
組織は、合理化され、法令に準拠する、協調的な取り組みを必要とします
統合プラットフォームでアートワークを一元管理
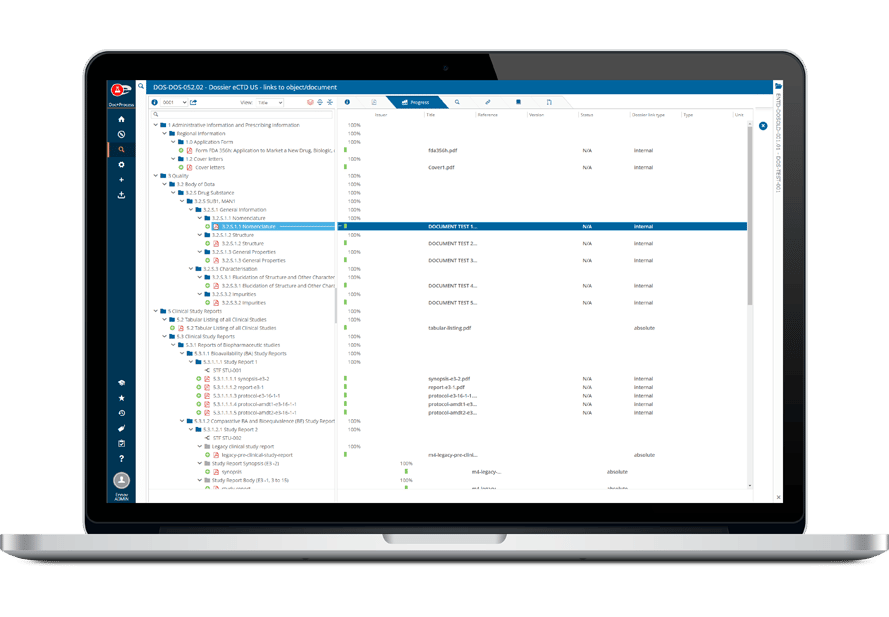
Ennov Artworkは、コンテンツ管理に特化した強力なツール群を用意し、コンテンツの項目化、注釈、コメント、ピクセル単位での比較を可能にします。
バージョン間の変更はっきりと区別でき、グラフィック・コンテンツとのやり取りは直感的な操作で行えます。
高度なバージョニング、注釈、署名ツールを一体化することで、Ennov Artworkは関係者全員が最新のファイルで作業できるようにし、ミスの発生リスクを削減し ます。このプラットフォームはあらかじめ設定されているので、操作しやすく、生産性が向上し、ユーザーの導入を後押しします。
Ennov Artworkは、最初のリクエストから最終承認まで、アートワークのライフサイクル全体を合理化し、完璧な追跡可能性と21 CFR Part 11やEU GMP Annex 11などの規制基準への遵守を保証します。
自動化されたワークフローとインテリジェント・ツールにより、プロジェクト管理が効率化され、TTM(time to market)を短縮します。
他のEnnovコアモデルとのシームレスな統合により、規制、申請、品質、供給データの統合リポジトリを実現するため、全体的なデータの整合性と一貫性が強化さ れます。
生産性と効率性の向上
Ennov Artworkの直感的なユーザーインターフェースと効率的な検索機能により、社員は必要な文書をすばやく見つけてアクセスすることができます。
このプラットフォームでは、ドキュメントに瞬時にアクセスでき、度重なるダウンロードは不要なため、チームは最新ファイルで作業できるようになります。
統合されたドキュメントテンプレート、堅牢なリビジョン管理、ドキュメントの自動命名および自動ナンバリング、電子署名機能により、アートワークファイルを時間どおりに作成し、規制基準に準拠させることができます。
複数のビジネスプロセスを管理
Ennov Artworkは、新製品の発売、リブランディング、規制の改正など、さまざまなアートワーク・プロジェクトに対応しています。
その高度な設定能力とEnnovの他のコアモデルとのシームレスな統合は、優れた運用性を実現する包括的なツールセットを提供します。
Ennov ArtworkはFDAの21 CFR Part 11要件(電子署名、監査証跡、記録管理)に完全に準拠しており、製薬、バイオテクノロジー、動物医療、医療機器などの規制産業に最適です。
中核的な機能
- アクセシビリティと使いやすさを追求した、100%WEBベースのプラットフォームは、グローバルな規制に対応する構成可能なテンプレート
- 完全準拠を実現するライフサイエンス分野のエキスパート向けソリューション
- シームレスなデータ管理を実現する統合プラットフォーム
- すべての規制活動に対する包括的な監査証跡
主な特長
- メタデータ付きの動的アートワーク・ファイル
- マルチメディアの管理と閲覧:JPEG、PDF、MP4、MP3、HTML
- バージョンアップと比較
- 直感的なユーザーインターフェースで検索と操作が簡単
- 規制上の節目に関する自動通知
- コンプライアンス管理に対応する自動ワークフロー
- 電子署名とSSO
Ennovを選ぶ理由
Ennovを信頼する数百社の顧客企業
20年以上にわたるライフサイエンス分野におけるソフトウェアソリューションの提供経験
ライフサイエンス分野の顧客は250社以上、その他の業界も多数
最新のアーキテクチャとインタフェース
WEBベース100%. 高い拡張性. ユーザー重視の設計
お客様の成功のために
顧客満足度が非常に高く、98.5%のプロジェクトが期限内、予算内に納品されている
お客様の選択の自由を尊重
クラウドベースまたはオンプレミスでの導入が可能
配置オプションの切り替えはいつでも可能
お客様の自主性を尊重
システム構成および管理に関するITスキルは不要
セキュリティの向上とパフォーマンスの最適化
データはローカルに保存されるが、柔軟性に富む。シングルテナントであるため、業務の中断を最小限に抑えられる

クラウドベースまたはオンプレミス

マルチプラットフォーム

