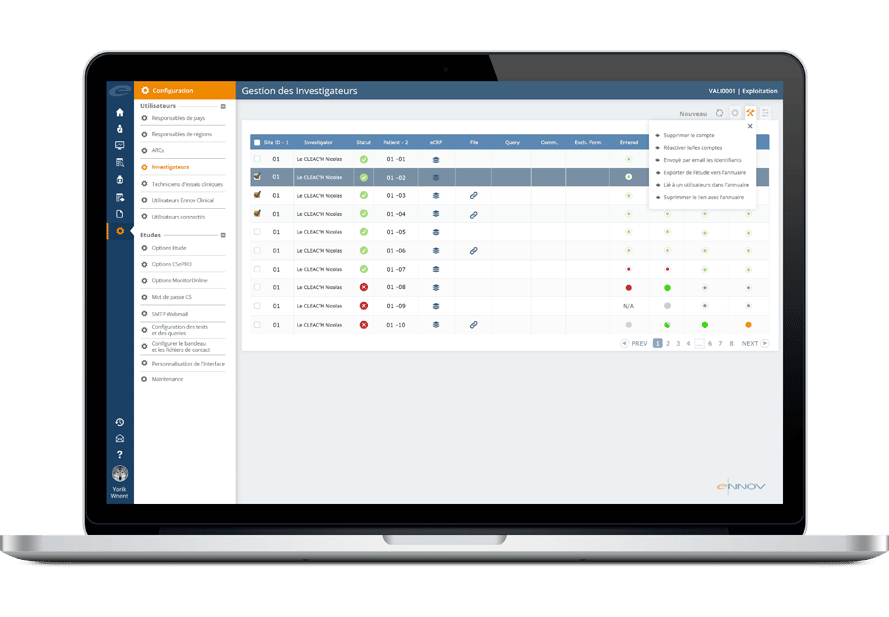
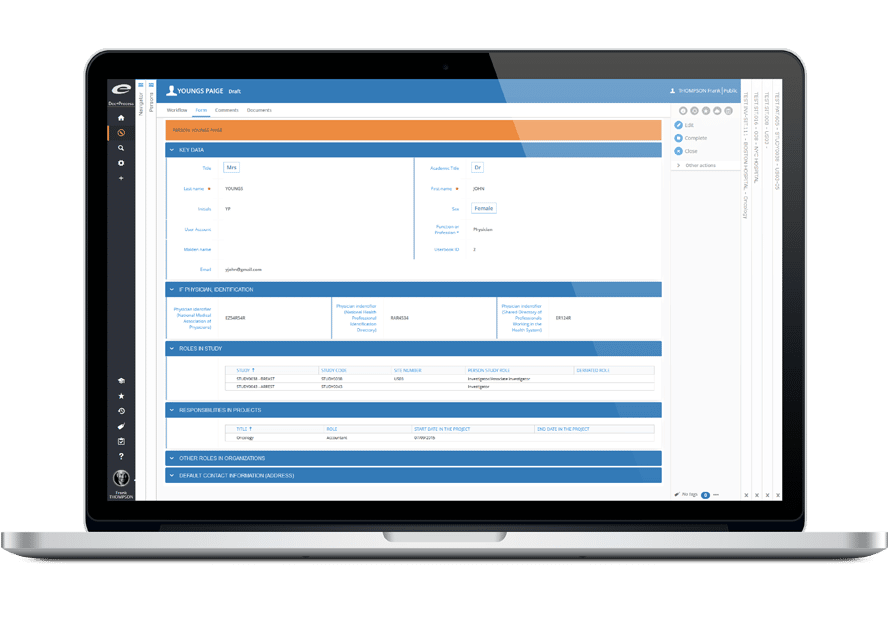
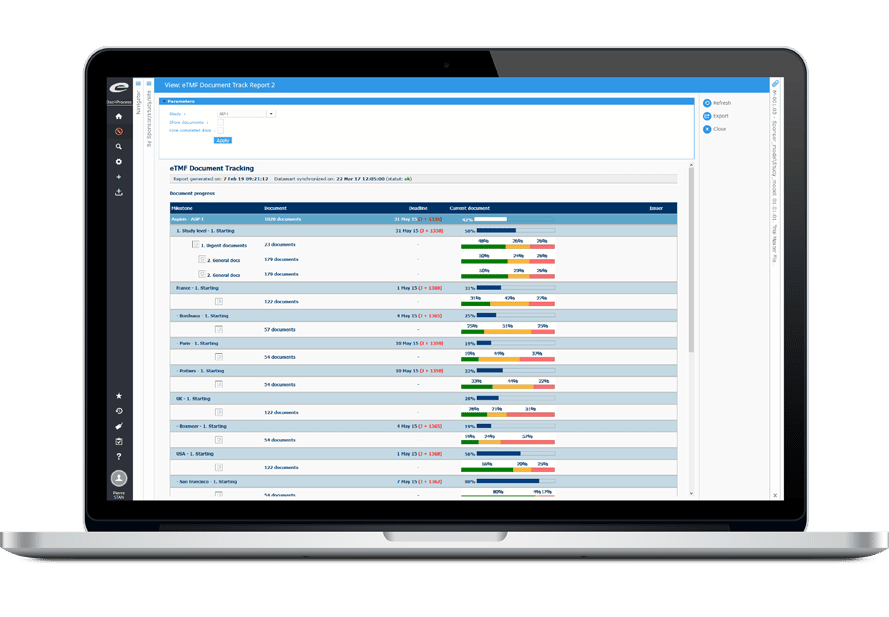
Ennov CLinical suite
MACRO
臨床試験管理ソフトウェア
- ICH 臨床試験の実施基準に準拠
- 親身で迅速なサポートチーム
- 迅速かつ容易なデータ収集のための即時応答検証機能
- スムーズなデータ管理のためのデータ検証、明確化、レポート、アラート
お客様のインフラに合わせたクラウドベースまたはオンサイトでのインストール
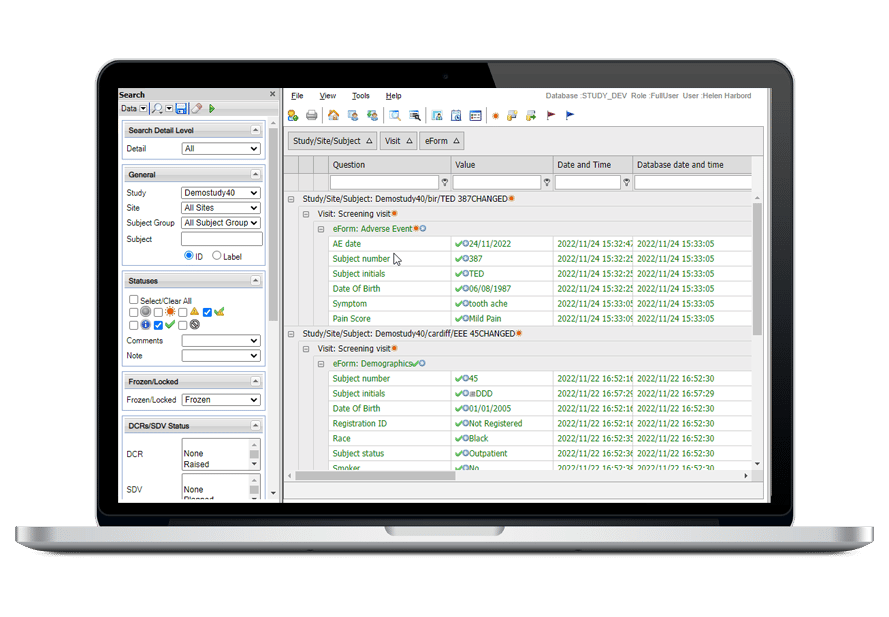
MACRO EDC
The MACRO Advantage
MACRO EDCは、データ整合性、データ管理、およびコンプライアンス機能を提供するため、お客様に有益です。
MACROの使いやすいシステムにより、ユーザーは対象データの入力、追跡、報告を迅速に行い、分析のために正確で信頼性の高いデータを収集することができます。
職員およびソフトウェアは、臨床試験実施基準 (GCP) に
従って監査され、ヒトを対象とする臨床試験の設計、実施、記録および報告に関する国際的に認められた倫理的および科学的品質基準に準拠していることを実証します。
MACROのユーザーは、被験者の権利、安全性および健康
が保護され、臨床試験の結果が信頼性が高く正確であることを保証できます。
MACROはあらゆる臨床試験段階の研究に適しているほ
か、あらゆる規模の試験に拡張可能です。
小規模、中規模、または大規模な組織で、単一施設試験や多国籍試験に使用できます。
MACROの特長
コンプライアンス・監査
- MHRAによる監査に合格
- ICH 臨床試験の実施基準に準拠
- EU臨床試験指令およびヒト用医薬品規則に準拠
- FDA 21 CFR Part IIに準拠
- ISO27001認証データセンター
- 監査ログ・監査証跡
- ダブル・タイムスタンプ
治験デザイン
- デザインからドラッグ・アンド・ドロップ
- ページ・レイアウトのフルコントロール
- 学習要素を簡単に再利用できる独自のライブラリを構築
- 訪問、フォーム、質問を条件付きで有効化
- 高度な計算と導き出し
- 柔軟で強力な編集
- テストとトレーニング環境
- 繰り返される質問表
- 様々な質問の種類と検査正常範囲
データ入力
- オンラインデータ入力
- データ入力時の値の即時検証
- 登録時の検証チェックを再評価
- 派生値の自動計算
- 電子署名と承認
- アラート、メッセージ、リマインダー
- 表示ステータスの概要
- MedDRA統合による臨床コーディング
データ管理
- 事前定義や自分仕様にされたレポート
- SPSS、SAS、STATA、CSVなどのエクスポートフォーマットを選択可能
- 統合されたデータの明確化とソースデータの検証プロセス
- 柔軟なデータベース・ロック機能
- データのインポートとエクスポート
- データアーカイブ
- イベントベースのルールと自動アラート
システム管理とセキュリティ
- データベースの作成、保存、管理
- 権限セットを使用して柔軟なユーザーロールを定義
- 治験と施設それぞれの組み合わせごとにユーザーロールを割り当て
- パスワードのプロパティ設定
- 柔軟なタイムアウト
- システム活動の監視
- セキュア・ソケット・レイヤー暗号化とデジタル署名、転送中のデータを保護する内蔵ブラウザー技術
- お客様のシステムとの統合用のパワフルなAPI
カスタマーサポート
- 重要な問題への4時間以内の対応
- 包括的なヘルプシステム
- 新機能の紹介ビデオ
- 対面式トレーニング
- ベストプラクティスを共有するためのオンライン・カスタマー・グ ループ
- 年次ユーザーグループ会議
カスタマーサポート : https://extranet.ennov.com
Ennov Clinical
臨床試験情報の取得と管理のための包括的な
ソリューション
Ennov Clinical Suiteは、臨床データ管理アプリケーション(EDC、RTSM、ePRO)と臨床試験管理アプリケーション(CTMS、eTMF)で構成されており、クラウドまたはオンプレミスで展開可能です。
Ennovを選ぶ理由
Ennovを信頼する数百社の顧客企業
20年以上にわたるライフサイエンス分野におけるソフトウェアソリューションの提供経験
ライフサイエンス分野の顧客は250社以上、その他の業界も多数
最新のアーキテクチャとインタフェース
WEBベース100%. 高い拡張性. ユーザー重視の設計
お客様の成功のために
顧客満足度が非常に高く、98.5%のプロジェクトが期限内、予算内に納品されている
お客様の選択の自由を尊重
クラウドベースまたはオンプレミスでの導入が可能
配置オプションの切り替えはいつでも可能
お客様の自主性を尊重
システム構成および管理に関するITスキルは不要
セキュリティの向上とパフォーマンスの最適化
データはローカルに保存されるが、柔軟性に富む。シングルテナントであるため、業務の中断を最小限に抑えられる

クラウドベースまたはオンプレミス

マルチプラットフォーム