ENNOV CLINICAL SUITE
Ennov IMPACT
IMPACTで臨床試験を効率的に管理
- 臨床試験を管理するすべての組織をサポート
- 単純なものから複雑なものまで、すべての治験タイプを管理
- モバイル監視アプリを含む総合的な治験実施施設の監視
- 堅固な治験責任医師の支払い管理
- タスク管理およびアラートの自動化
- すぐに利用できるようBOXを実装
はじめに
Ennov IMPACTは臨床試験管理を合理化するために設計された包括的な CTMSソリューションです。治験実施施設の管理に比類ない可視性と効率性を提供します。業界で実証されたこのツールは、トライアルの質、コンプライアンス、生産性を高め、治験の成果を高める最も効果的なツールを治験チームに提供します。
臨床試験管理の課題
臨床試験を取り巻く環境は複雑さを増しており、薬事規制に対する要求も高まり、効率的な試験管理が求められています。
組織は、コンプライアンスの維持、膨大なデータ管理、タイムリーで正確な臨床試験の実施といった課題に直面しています。堅牢なCTMSがなければ、これら の課題はコスト増、遅延、データ品質の低下につながり、最終的には臨床試験の成功に影響を及ぼします。
ソリューションの概要
Ennov IMPACTが優れている理由
Ennov IMPACTは、試験リスクを低減し、複数のレベルでコンプライアンスを確保する堅牢な機能を備えています。
予定された募集と実際の募集と登録を比較検討することにより、修正計画のタイムリーな実施を可能にし、コストのかかる遅延を回避します。
カスタマイズ可能なチェックリストと主要なサイト情報から、モニターはクリティカルタスクに集中でき、電子署名によるエンドツーエンドのワークフローを通じてコンプライアンスを確保できます。
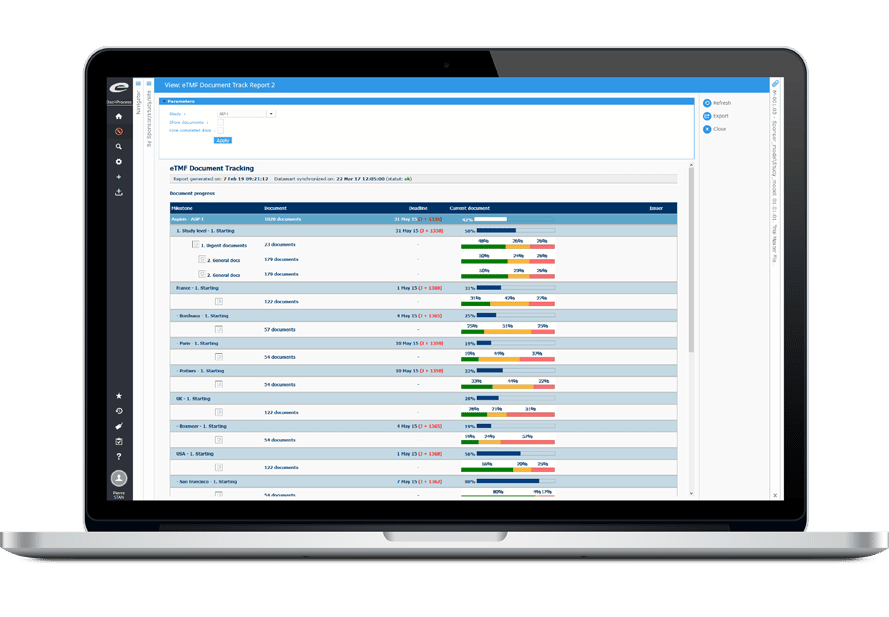
高度な分析とレポーティング
Ennov IMPACTは、過去に実施されたすべての臨床試験のオペレーショナルデータを一元管理し、効率的な交差試験を促進します。
これにより、新たなトレンドの特定、治験実施施設選定の最適化、過去の成功例に基づく現実的な計画の作成が可能になり、意思決定と治験のパフォーマンスが向上します。
導入
当社の導入および開発チームは、豊富な経験と専門知識を備えています。
統合
Ennov IMPACTを既存のeクリニカルシステムにシームレスに接続することで、完全なデータ品質を確保し、生産性を高めます。
EDC、IRT、eTMFシステムとのセルフサービスによる標準的な統合に対応しています。
また、財務台帳、ファーマコビジランス、プロジェクト管理など、他のシステムとのカスタム統合も可能です。
中核的な機能
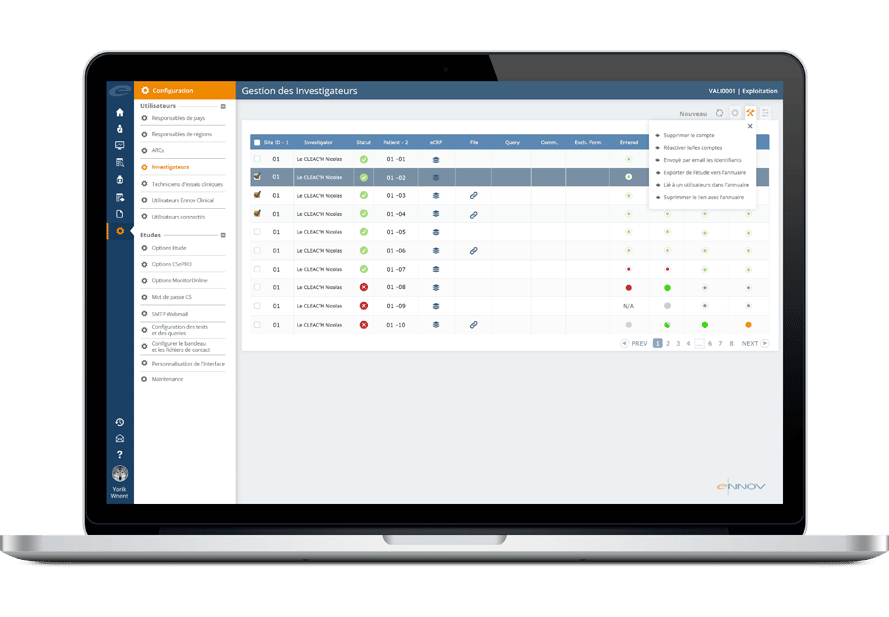
- モバイルアプリを含む包括的な監視
- 問題とプロトコル逸脱の追跡
- 治験責任医師の支払い管理
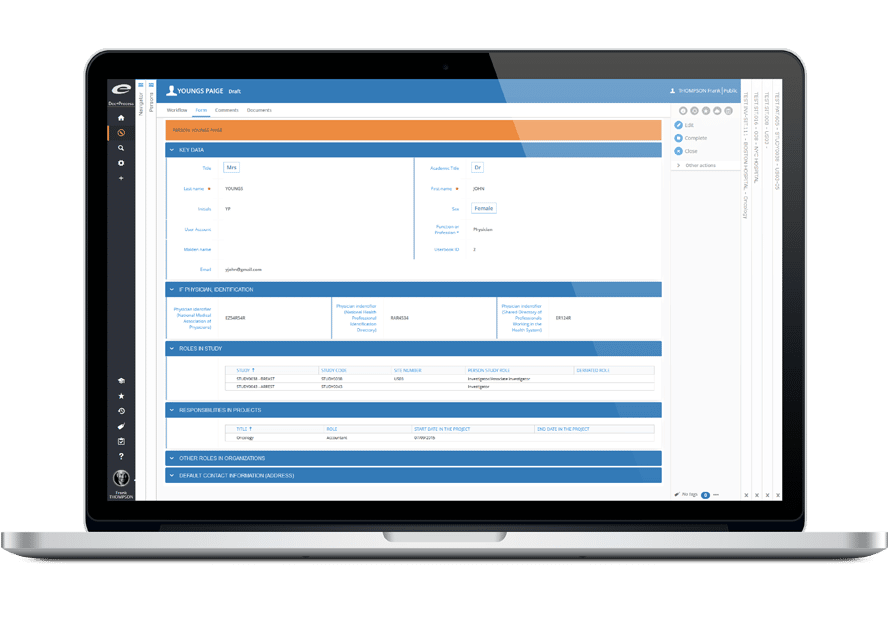
- 組織・人事の総合名簿
- 高度な設定が可能
- 被験者募集の追跡
主な特長
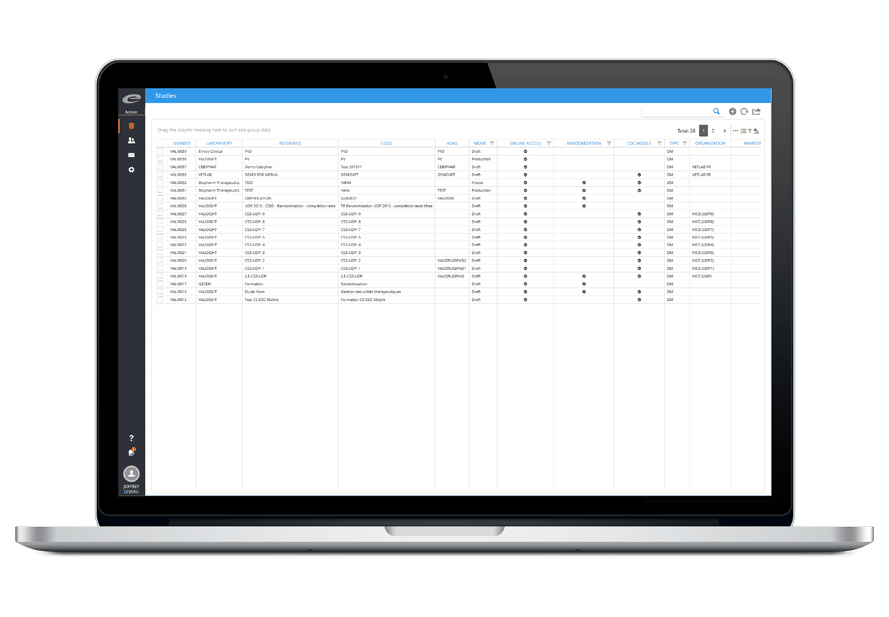
- 一元化されたグローバル・データベース
- 箱から出してそのまますぐ使える
- 統合ワークフロー
- 直感的なユーザーインターフェース
- セルフサービスによる統合が可能
- 100%ウェブベース
- 21 CFR Part 11 コンプライアンスに対応
Ennov Clinical
臨床試験情報の取得と管理のための包括的な
ソリューション
Ennov Clinical Suiteは、臨床データ管理アプリケーション(EDC、RTSM、ePRO)と臨床試験管理アプリケーション(CTMS、eTMF)で構成されており、クラウドまたはオンプレミスで展開可能です。
Ennovを選ぶ理由
Ennovを信頼する数百社の顧客企業
20年以上にわたるライフサイエンス分野におけるソフトウェアソリューションの提供経験
ライフサイエンス分野の顧客は250社以上、その他の業界も多数
最新のアーキテクチャとインタフェース
WEBベース100%. 高い拡張性. ユーザー重視の設計
お客様の成功のために
顧客満足度が非常に高く、98.5%のプロジェクトが期限内、予算内に納品されている
お客様の選択の自由を尊重
クラウドベースまたはオンプレミスでの導入が可能
配置オプションの切り替えはいつでも可能
お客様の自主性を尊重
システム構成および管理に関するITスキルは不要
セキュリティの向上とパフォーマンスの最適化
データはローカルに保存されるが、柔軟性に富む。シングルテナントであるため、業務の中断を最小限に抑えられる

クラウドベースまたはオンプレミス

マルチプラットフォーム