ENNOV CLINICAL SUITE
Ennov DataLabs
Ennov DataLabsで臨床試験を変革:高い信頼性柔軟効率的
- TSDV(Targeted SDV)で効率的にリソースを集中させる
- 堅牢なAPIを通じて他社システムとシームレスに統合
- リアルタイムの安全報告で患者様の安全性を高める
- 急な治験構築に使い勝手のよいインターフェース
- 100%ウェブベース・ソリューション
はじめに
Ennov DataLabsは、柔軟性、信頼性、効率性に優れた 電子データ収集(EDC)システムで、臨床データ管理に革命をもたらします。
バイオ医薬品のスポンサー、CRO、医療機器メーカー向けに設計されたEnnov DataLabsは、優れたユーザビリティと機能性を実現し、試験デザインからデータ報告までのライフサイクル全体をサポートします。
臨床試験管理の課題
臨床試験管理は、厳しい規制要件への対応から膨大なデータの管理まで、複雑さをはらんでいます。
組織はしばしば、データの品質維持、患者の安全確保、資源配分の最適化に苦慮しています。
臨床試験管理における非効率性は、コスト増、遅延、そして
患者の安全性に対する潜在的なリスクにつながる可能性があります。
堅牢なEDCシステムがなければ、これらの課題はますます増加し、臨床試験の全体的な成功と信頼性に影響を 及ぼすことになります。
ソリューションの概要
Ennov DataLabsは、高度な機能と性能を備えた包括的なスイートで、これらの課題に正面から挑みます。
Ennov DataLabsが優れている理由
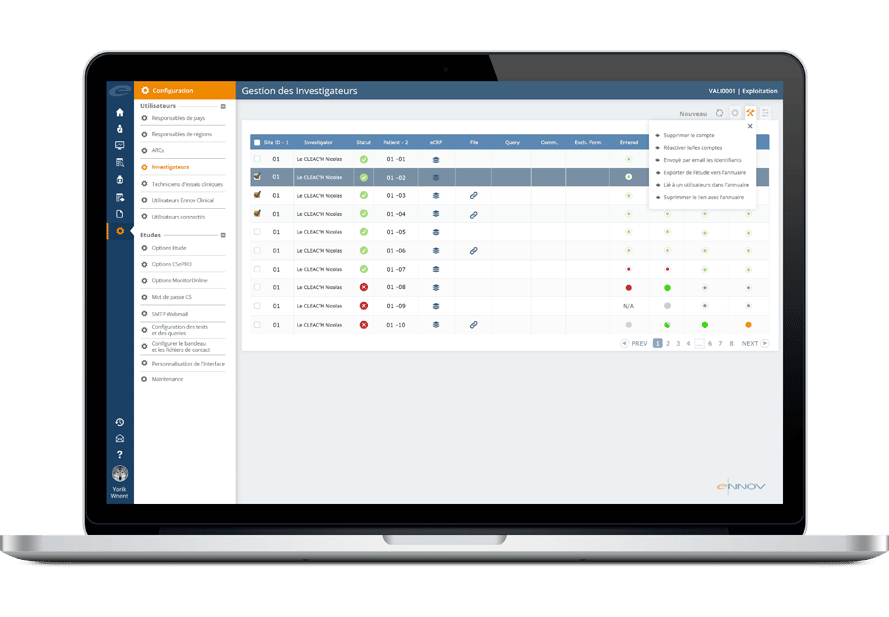
EnnovDataLabsは、ターゲットを絞ったソースデータ検証 (SDV) 機能を備えており、最も必要とされる場所での監視作業に焦点を当て、オンサイトの監視作業と関連コストを削減します。
これにより、治験チームはデータに基づいた意思決定を行うことができ、効率と資源配分を向上させることができます。
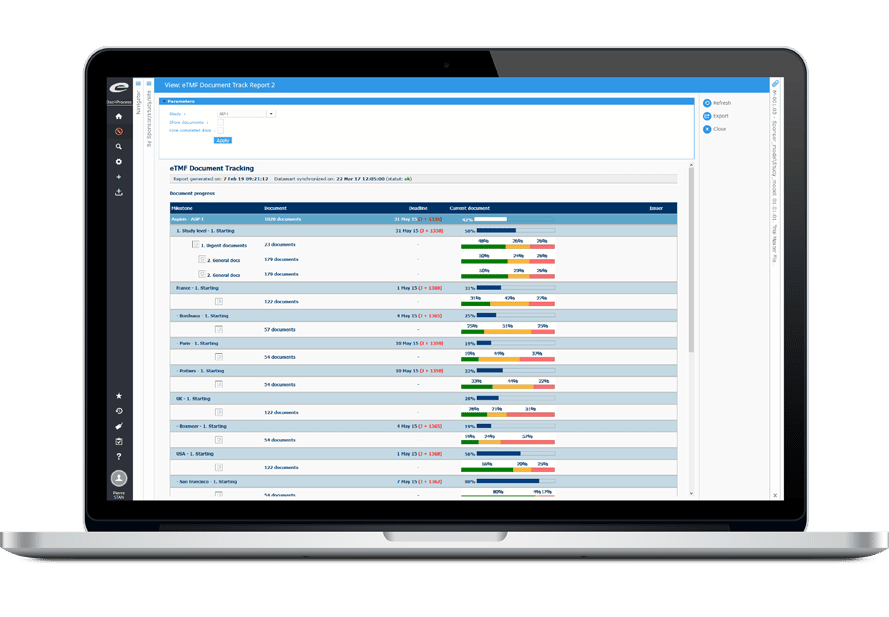
高度な分析とレポーティング
このプラットフォームには、ダッシュボードやアドホックレポート機能などの堅牢なレポートツールが搭載されており、試験指標を明確に可視化し、戦略的意思決定をサポートします。
統合された安全性レポートにより、重要な安全性イベントをリアルタイムに通知し、患者の安全性とコンプライアンスを向上させます。
導入・統合
EnnovDataLabsは、シンプルなユーザーインターフェースとドラッグ&ドロップによる治験構築機能により、迅速かつ直感的な治験デザインを容易にします。
その堅牢なAPI Webサービスは、さまざまなシステムとのシームレスな統合を可能にし、総合的なデータ管理を保証し、不整合を排除します。
中核的な機能
- ターゲットソースデータ検証 (TSDV)
- リアルタイムの安全報告
- 堅牢なAPIによるシームレスな統合
- 直感的な治験構築作成ツール
- 総合的なデータ管理とレポート作成
主な特長
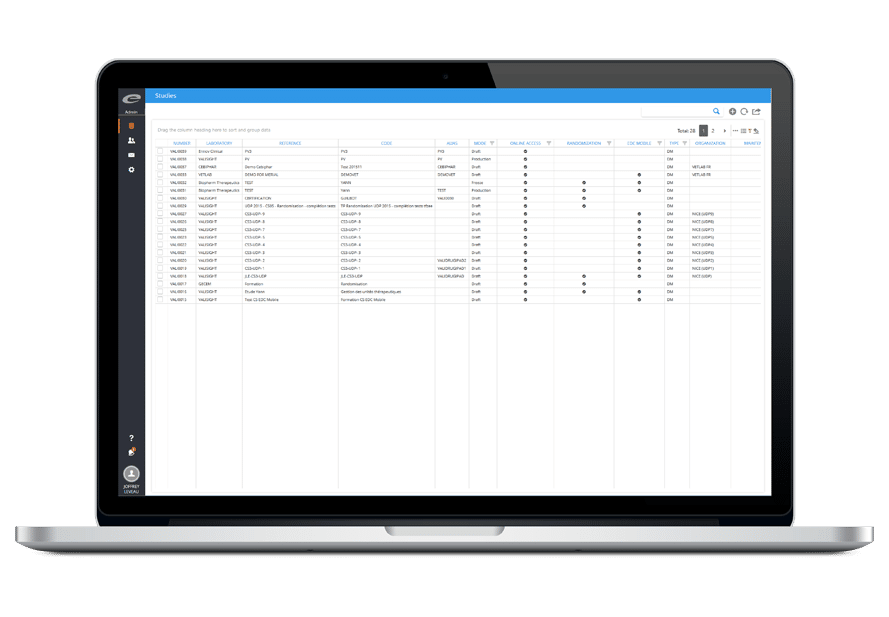
- 一元化された治験デザイン対象のリポジトリ
- データ検証のためのビルトイン編集チェック
- 治験構築のためのドラッグ・アンド・ドロップ・インターフェース
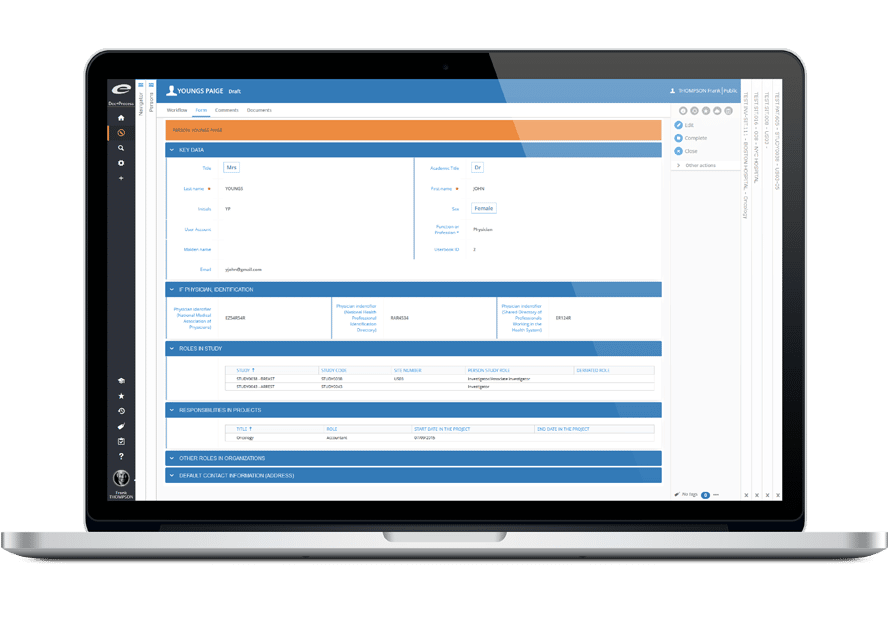
- 患者別CRFの動的生成
- 治験デザイン変更点の視覚的比較
- 21 CFR Part 11に準拠したウェブベース
Ennov Clinical
臨床試験情報の取得と管理のための包括的な
ソリューション
Ennov Clinical Suiteは、臨床データ管理アプリケーション(EDC、RTSM、ePRO)と臨床試験管理アプリケーション(CTMS、eTMF)で構成されており、クラウドまたはオンプレミスで展開可能です。
Ennovを選ぶ理由
Ennovを信頼する数百社の顧客企業
20年以上にわたるライフサイエンス分野におけるソフトウェアソリューションの提供経験
ライフサイエンス分野の顧客は250社以上、その他の業界も多数
最新のアーキテクチャとインタフェース
WEBベース100%. 高い拡張性. ユーザー重視の設計
お客様の成功のために
顧客満足度が非常に高く、98.5%のプロジェクトが期限内、予算内に納品されている
お客様の選択の自由を尊重
クラウドベースまたはオンプレミスでの導入が可能
配置オプションの切り替えはいつでも可能
お客様の自主性を尊重
システム構成および管理に関するITスキルは不要
セキュリティの向上とパフォーマンスの最適化
データはローカルに保存されるが、柔軟性に富む。シングルテナントであるため、業務の中断を最小限に抑えられる

クラウドベースまたはオンプレミス

マルチプラットフォーム