Ennov regulatory suite
Ennov InSight Registration
規制データを価値ある洞察に変換
- エンドツーエンドの規制情報管理
- 合理化された申請書類計画と書類提出の追跡
- 包括的な製品および登録管理
- Microsoft Power BIとの統合による高度な分析
- 100%ウェブベースの拡張可能なソリューション
- 世界中の規制コンプライアンス要件に対応
- 強化されたデータセキュリティと操作しやすいインターフェース
はじめに
Ennov InSight Registrationは、規制情報管理(RIM)に革命を起こすべく、20年にわたるお客様からのフィードバックから磨き上げられた先進のソリューションです。
規制データを効率的に管理、分析、活用し、コンプライアンスを確保しながら貴重な知見を引き出せるようにします。
Ennov InSight Registrationを利用することで、企業は規制プロセスを合理化し、試験完了から申請までの時間を短縮し、最終的には患者様の生活を向上させる新治療法の承認を加速させることができま
す。
規制情報管理の課題
ライフサイエンス業界におけるコンプライアンスには、膨大な量のデータを処理する必要があります。この処理には膨大な時間がかかります。
組織は、広範な規制コンテンツの管理、申請書類の追跡、タイムリーで正確な情報処理を確保しながら、コンプライアンスを維持するという課 題に直面しています。
堅牢なRIMシステムがなければ、これらの課題は、コンプライアンス違 反、非効率性、コスト増、治療薬の市場投入の遅れによる収益損失につながる可能性があります。
規制要件の複雑さとシームレスなデータ統合の必要性は、これらの問題をさらに深刻化させるため、組織は包括的なRIMソリューションを採用することが極めて重要になります。
ソリューションの概要
Ennov InSight Registrationは、規制プロセスを簡素化・合理化するエンドツーエンドのRIMプラットフォームを提供することで、こうした課題に対処します。
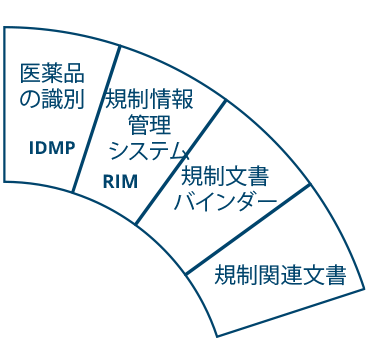
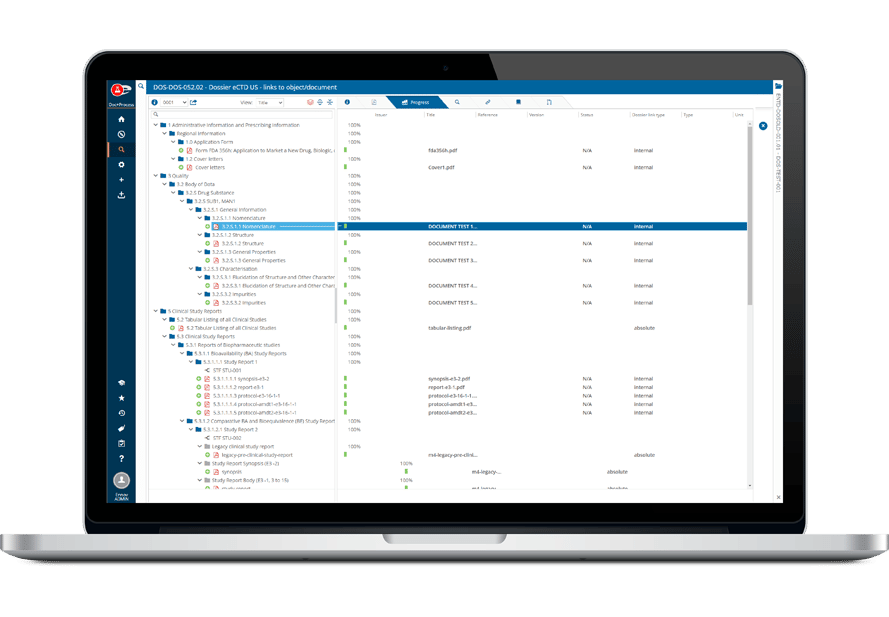
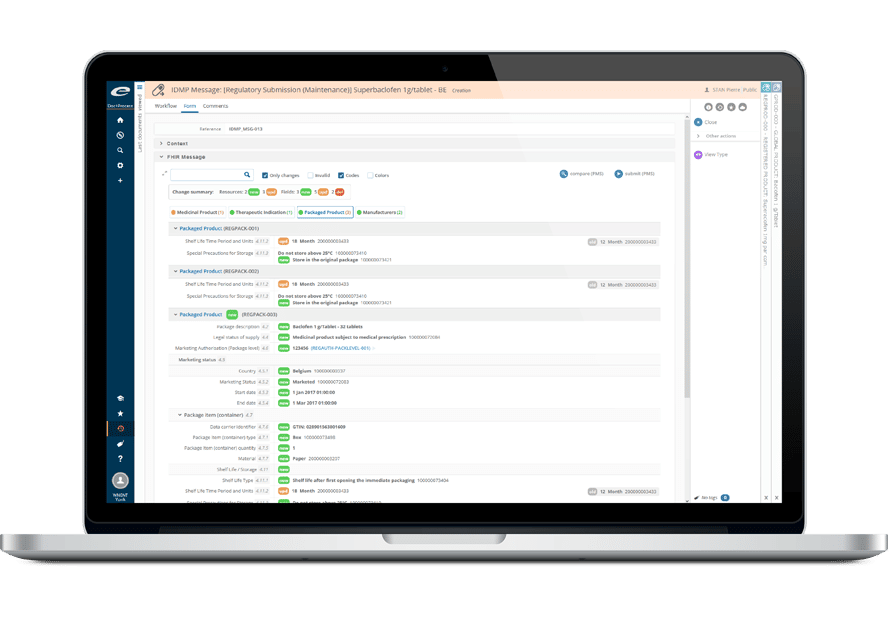
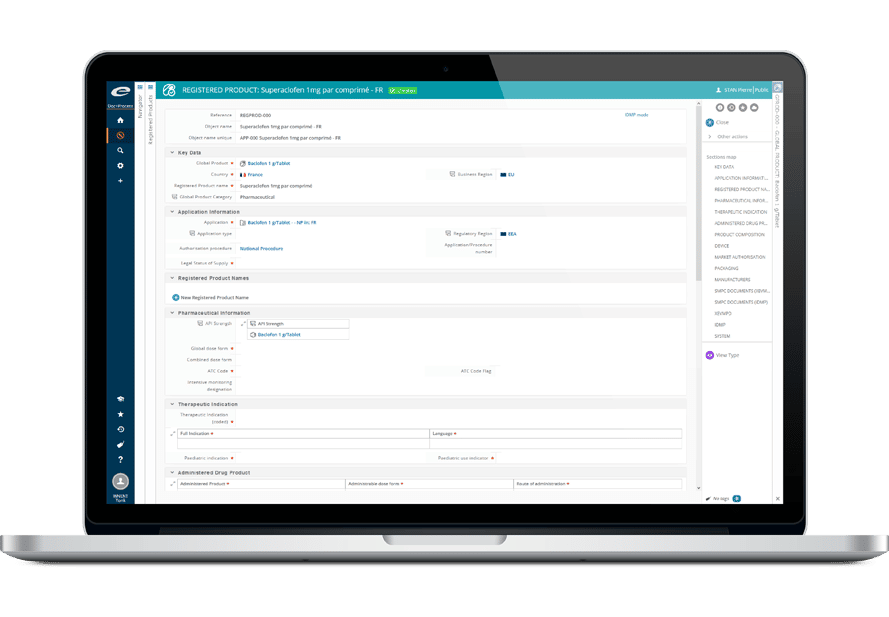
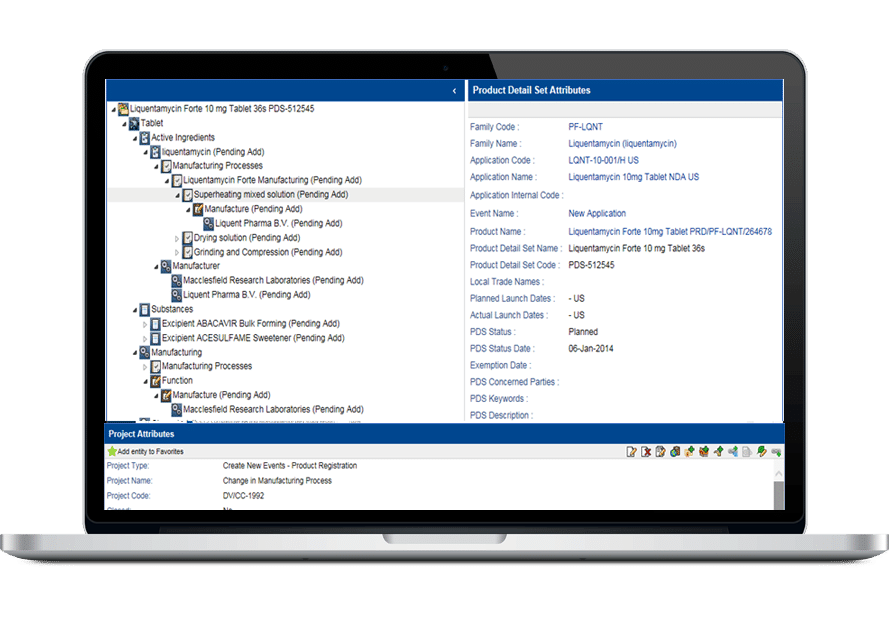
その堅牢なデータ管理構造は、書類計画、申請管理から製品・登録追跡まで、規制全体のライフサイクルをサポートします。
Microsoft Power BIと統合されたこのプラットフォームは、高度な分析とレポーティング機能を提供し、企業が規制データから実用的な洞察を導き出すことを可能にします。
さらに、柔軟なAPIにより、Ennov InSight Registrationは規制エ コシステムの中心的存在となり、他社システムとのシームレスな統合を可能にします。
Ennov InSight Registrationのウェブベースのインターフェースは、定評ある操作性を備え、使いやすさとアクセシビリティを保証します。また、新興企業から多国籍企業まで、あらゆる規模の企業ニーズに応えるデザインとなっています。
中核的な機能
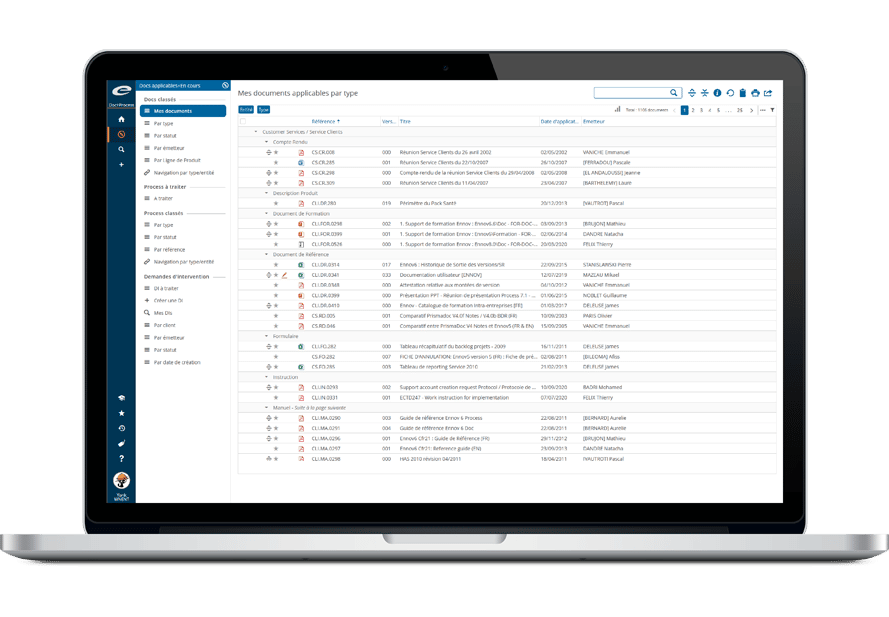
- エンドツーエンドの規制情報管理
- 書類計画・申請書の追跡
- 包括的な製品および登録管理
- Microsoft Power BIとの統合による高度な分析
- ワークフローの自動化とプロセスの効率化
- 安全なウェブベースのプラットフォーム
主な特長
- 一元化された規制データリポジトリ
- 既存のコンテンツ管理システムとのシームレスな統合
- 使いやすさを追求した直感的ユーザーインターフェース
- 包括的で設定可能なデータモデル
- リアルタイム分析およびレポートダッシュボード
- 世界中の規制基準に準拠
- 組織のニーズに合わせて拡張可能
Ennov Regulatory
世界最高水準の規制コンテンツと情報管理
Ennov Regulatory Suite は Ennov Doc、Ennov Dossier、Ennov Process の機能と柔軟性を組み合わせ、登録目標の初期計画から製品の廃止まで、規制当局の製品ライフサイクル全体にわたってサポートするものです。Ennov Regulatory Suiteは、規制当局の活動計画、製品登録管理、資料作成、資料管理などにおいて非常に有用なツールです。
Ennovを選ぶ理由
Ennovを信頼する数百社の顧客企業
20年以上にわたるライフサイエンス分野におけるソフトウェアソリューションの提供経験
ライフサイエンス分野の顧客は250社以上、その他の業界も多数
最新のアーキテクチャとインタフェース
WEBベース100%. 高い拡張性. ユーザー重視の設計
お客様の成功のために
顧客満足度が非常に高く、98.5%のプロジェクトが期限内、予算内に納品されている
お客様の選択の自由を尊重
クラウドベースまたはオンプレミスでの導入が可能
配置オプションの切り替えはいつでも可能
お客様の自主性を尊重
システム構成および管理に関するITスキルは不要
セキュリティの向上とパフォーマンスの最適化
データはローカルに保存されるが、柔軟性に富む。シングルテナントであるため、業務の中断を最小限に抑えられる

クラウドベースまたはオンプレミス

マルチプラットフォーム