Ennov regulatory suite
Ennov InSight Publishing
簡単で効率的な薬事申請関連業務の合理化
- コンプライアンスに準拠した提出書類の作成を迅速化
- 4.0および電子申請を含むグローバルeCTD仕様に対応
- CROWおよびレポート発行のための総合的なペーパー機能
- 各地域における要件を合理化する統合ウィザード
- 優れたブックマークおよびリンクツール
- コンプライアンス確保のためのビルトイン検証チェック
- ユーザーが構成可能なランディング・ページとお気に入り機能
- 複数のドキュメントの場所からコンテンツをドラッグ&ドロップで簡単にリンク
はじめに
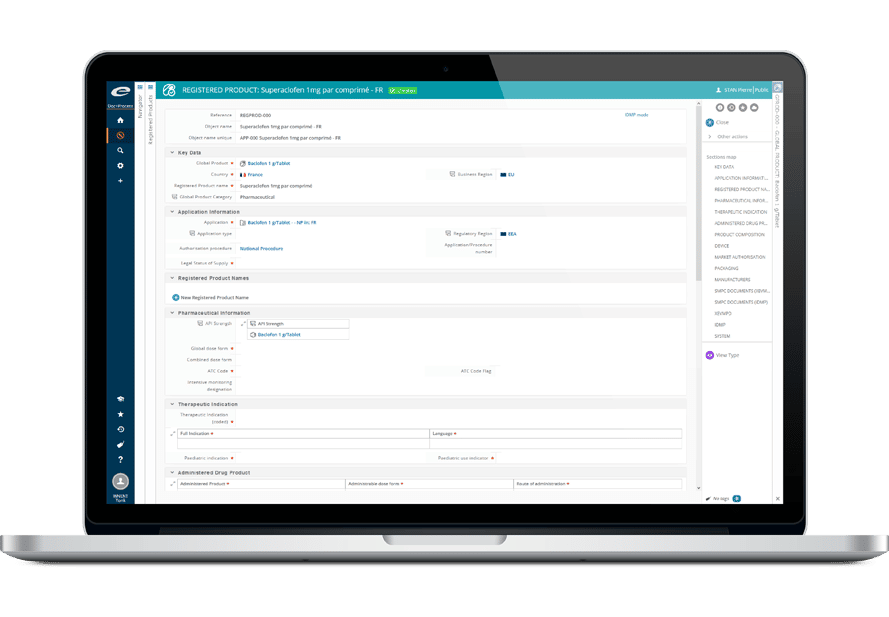
Ennov InSight Publishingは、薬事申請関連業務のプロセスを簡素化・合理化するために設計された包括的なソリューションです。
これにより、ライフサイエンス企業は、あらゆる規制
フォーマットで規制文書を作成、管理、提出することができ、各地域の要件に確実に適合することができます。
高度なツールと直感的なユーザーインターフェイスを備えているので、
InSight Publishingは、企業が納期を守り、最高水準のデータ品質を維持できるようサポートします。
薬事申請関連業務の課題
ライフサイエンス業界における薬事申請関連業務
(Regulatory publishing)は複雑で時間のかかる作業であり、細部にまで細心の注意を払い、さまざまな地域の基準に準拠する必要があります。
企業は多くの場合、複数のフォーマット管理、申請書類のライフサイクルを追跡、そしてすべての書類が最新の規制に適合するようにすることに苦慮しています。
効率的なシステムがなければ、これらの課題は、遅延、コスト増、潜在的なコンプライアンス問題につながり、最終的には製品の市場参入とライフサイクル管理の妨げ となり、収益の損失につながります。
ソリューションの概要:Ennov InSight Publishingが優れている理由
Ennov InSight Publishingは、その総合的な機能と操作性に優れたデザインにより、薬事申請関連業務の有力なソリューションとして注目されています。
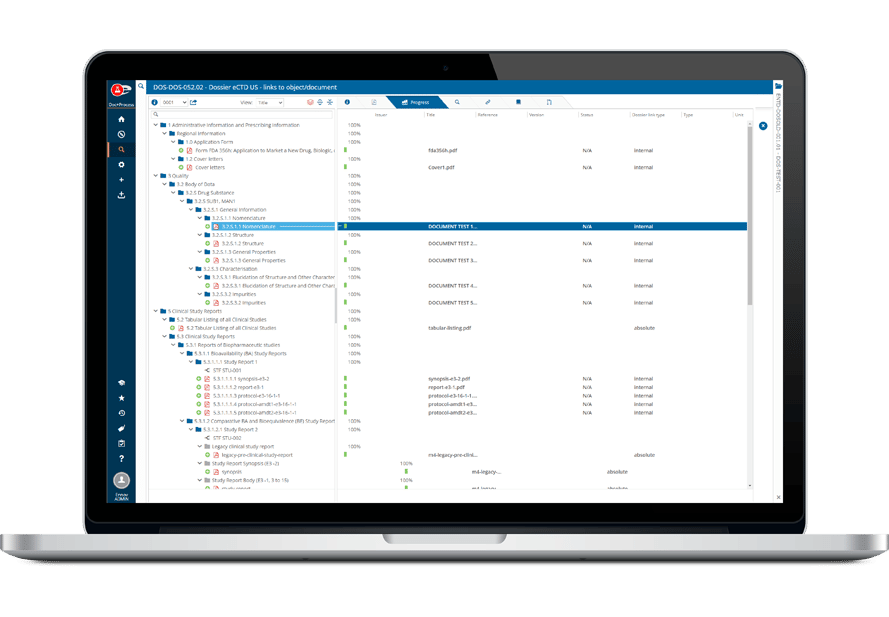
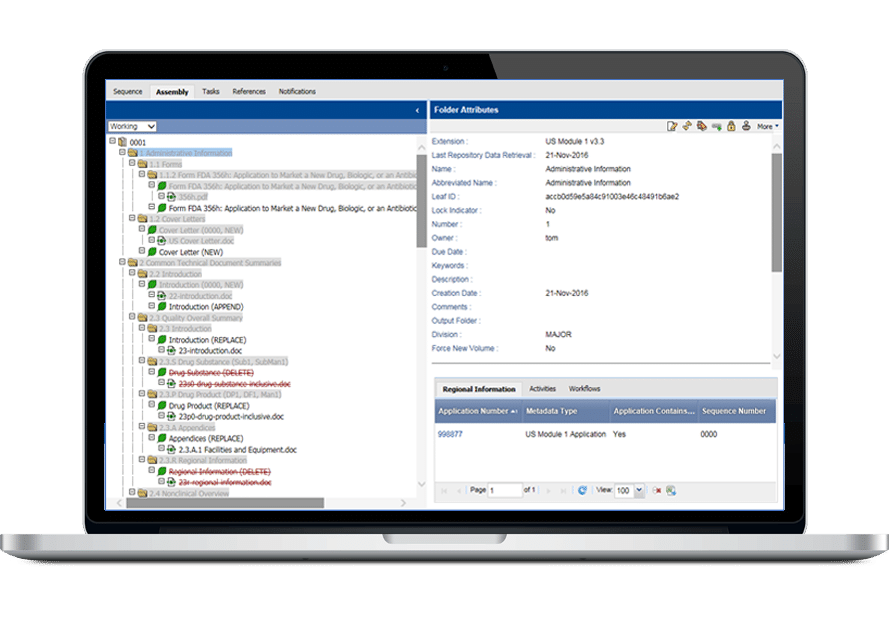
共通のインターフェイスで電子媒体(eCTDとNeeS)と紙媒体の申請に対応しているため、ユーザーは同じアセンブリから異なる地域向けの複数の申請を簡単に作成できます。
このプラットフォームに組み込まれたテンプレートとウィザードは、作成プロセスを通じてユーザーを案内し、ミスを減らし、地域規格への準拠を確実にします。
高度なツールとユーザーインターフェース
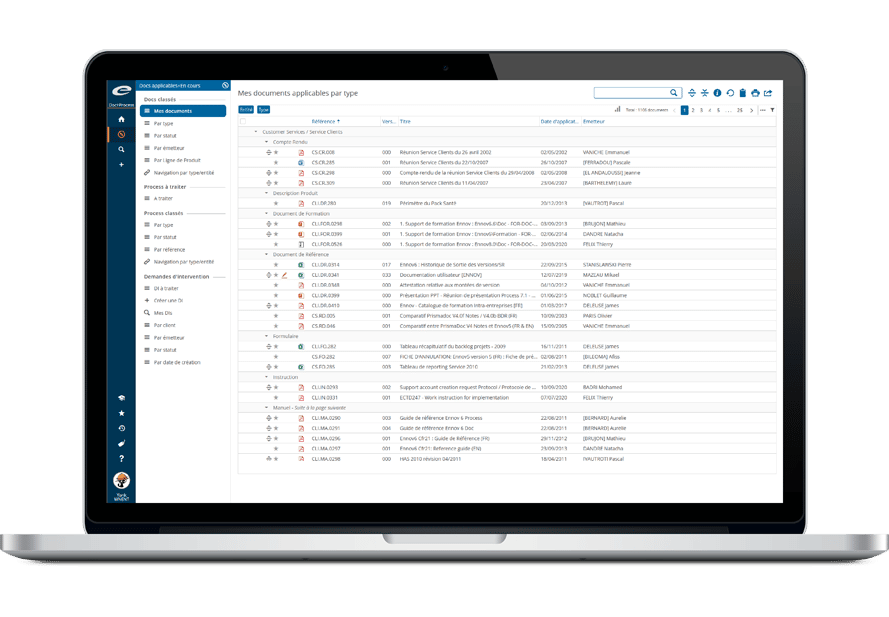
InSight Publishingは、洗練されたリンクとブックマー クツールを備え、申請書類を簡単に閲覧・整理することができます。
統合された検証ツールは、申請書類が必須要件を満たしているかを確認し、是正措置に関する包括的なインタラクティブレポートを提供します。
ユーザー設定が可能なランディングページとお気に入り機能により、ユーザーは自分の体験を自分仕様にすることができ、効率と使いやすさ を向上させることができます。
導入・統合
このプラットフォームは、VeevaやDocumentumのような既存の文書管理システムとシームレスに統合でき、ドラッグ・アンド・ドロップ機能で簡単にコンテンツを割り当てることができます。
InSight Publishingを支える専門家チームは、規制情報管理において100年以上の実績があり、貴社の ニーズに合わせた導入を成功に導きます。
中核的な機能
- コンプライアンスに準拠した申請書の作成を加速
- eCTD、NeeS、紙媒体での申請にグローバル対応
- 手順を追って説明するガイダンスのための統合ウィザード
- 優れたブックマークおよびリンクツール
- 全地域に対応したアセンブリ・テンプレートを内蔵
- ユーザー設定が可能なランディングページとお気に入り
- 文書管理システムとのドラッグ&ドロップ統合
主な特長
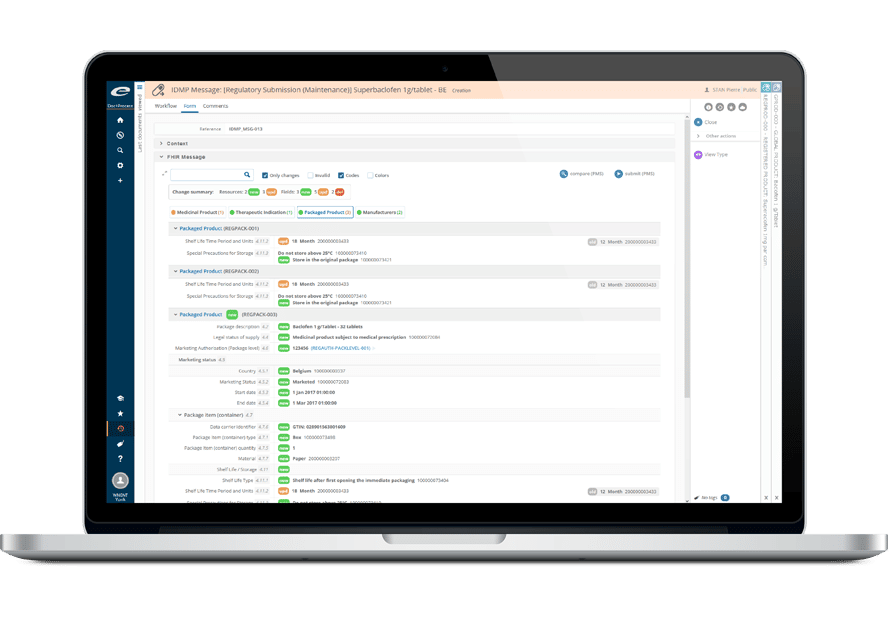
- 包括的で設定可能なデータモデル
- 反復パブリッシング機能
- eCTDおよびNeeS申請用検証ツール
- ICH STFおよび地域XMLファイルの作成
- 修正、補足、変更点の管理
- すべての申請タイプに共通のユーザー・インターフェース
- クエリ結果のエクスポート機能
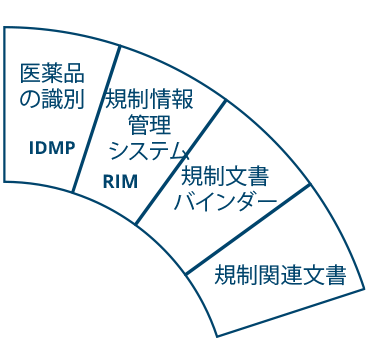
Ennov Regulatory
世界最高水準の規制コンテンツと情報管理
Ennov Regulatory Suite は Ennov Doc、Ennov Dossier、Ennov Process の機能と柔軟性を組み合わせ、登録目標の初期計画から製品の廃止まで、規制当局の製品ライフサイクル全体にわたってサポートするものです。Ennov Regulatory Suiteは、規制当局の活動計画、製品登録管理、資料作成、資料管理などにおいて非常に有用なツールです。
Ennovを選ぶ理由
Ennovを信頼する数百社の顧客企業
20年以上にわたるライフサイエンス分野におけるソフトウェアソリューションの提供経験
ライフサイエンス分野の顧客は250社以上、その他の業界も多数
最新のアーキテクチャとインタフェース
WEBベース100%. 高い拡張性. ユーザー重視の設計
お客様の成功のために
顧客満足度が非常に高く、98.5%のプロジェクトが期限内、予算内に納品されている
お客様の選択の自由を尊重
クラウドベースまたはオンプレミスでの導入が可能
配置オプションの切り替えはいつでも可能
お客様の自主性を尊重
システム構成および管理に関するITスキルは不要
セキュリティの向上とパフォーマンスの最適化
データはローカルに保存されるが、柔軟性に富む。シングルテナントであるため、業務の中断を最小限に抑えられる

クラウドベースまたはオンプレミス

マルチプラットフォーム