Recently, I reviewed the results of the Gens & Associates’ World Class RIM Survey. There is a wealth of great information on RIM investments, benefits, practices, and solutions. One of the six stated goals of the study was specifically to “Quantify industry short and long-term strategies for structured data submissions.” In this area, the survey uncovered that many sponsors are not well-prepared for upcoming structured submission requirements.
As most regulatory professionals in the industry know, IDMP is becoming closer to reality with each passing month and is surely the harbinger of greater structured data submissions. As a refresher, the draft EU Implementation Guidance v2 was released for comment in July, and the final version is expected in December 2020. Once this version 2 is released, a one-year implementation period for optional use of ISO IDMP will begin, followed by an additional year implementation period before ISO IDMP becomes mandatory.
However, despite this, it appears that for most organizations, the timeframe for reliably generating IDMP submissions once the implementation guide is published is either wholly unknown or almost two years distant. Even for companies with active or imminent IDMP projects, this is expected to take more than a year, if not closer to two.
Specifically, the survey asked sponsors to estimate how many months after the publication of the final EU Iteration 1 Implementation Guide until they expect to be able to make SPOR compliant submissions to EMA.
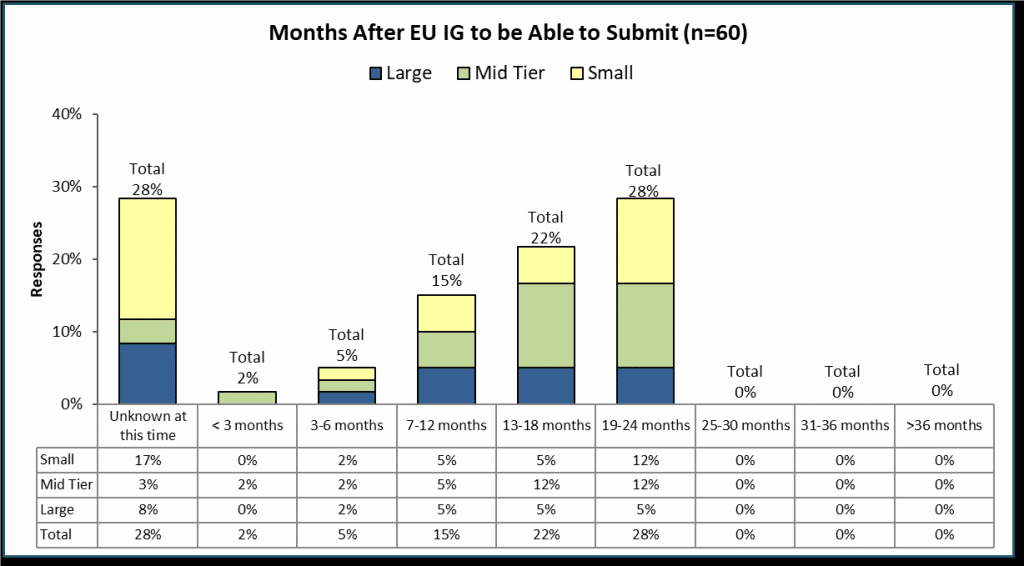
The survey results show that almost 30% of respondents feel it’s too early to predict when they will be ready. Clearly, most companies see this as a significant effort continuing after the publication of the next version of the EU Implementation Guide. Only 42% of sponsors appear to be confident that they will be ready comfortably in advance of the mandatory submission time (18 months or less).
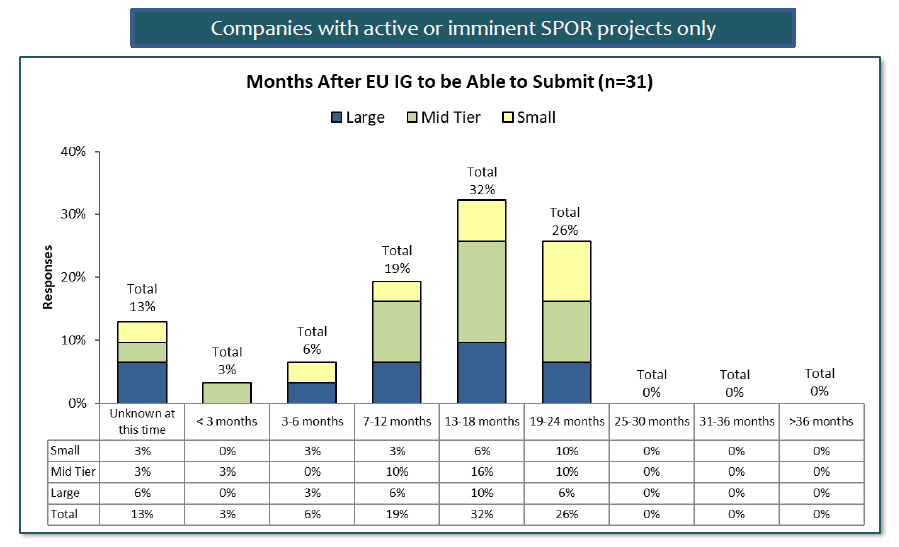
For companies with active or imminent SPOR initiatives, the picture is a little brighter. Estimates are mainly falling in the 7-24 month range for these companies, with most estimating in the 13-18 month range. But a surprising 26% feel that they will need almost all the allocated time – and that is without considering the 13% who are still not able to estimate when they will be ready.
But this data takes on a different perspective when viewed in contrast to how structured data submissions are done currently. According to the survey, the majority of companies are either outsourcing them or performing them manually, with only a bare minimum automating them with in-house tools. How will organizations be able to transition from manual and outsourced processes to handle IDMP? Are realistic time and resource estimates being prepared?
I suspect that IDMP requirements represent a far more rigorous standard than most organizations are used to delivering, and if those with more advanced projects are predicting a long project until go-live, where does that leave everyone else?
The survey indicates that 80% of top-performing organizations expect RIM to be the primary system supporting IDMP/SPOR, with the remaining 20% viewing RIM as a supporting system. This will require not only organizational changes and alignment but also technology partners who have a strong and innovative strategy in place.
Survey respondents also viewed Ennov as that strong partner, both terms in innovation and as a company garnering much industry interest for our IDMP strategy and approach. Our keen focus on providing innovative solutions is why many customers are looking at our IDMP solution, but also, with our full spectrum of capabilities, our RIM capabilities more broadly.
At Ennov, we have been preparing for IDMP since the creation of our RIM solution. Due to delays in initial Agency timelines, we decided to implement xEVMPD. As a result, we defined our solution knowing that first IDMP and then other structured data standards are coming, resulting in a framework that can handle any new data and process requirements almost entirely by configuration. This includes the management of multiple controlled vocabularies (with the challenge of their alignment), design of the data model and fields, and uses of flexible workflows for submission and management of the responses. Integrating with systems that would provide the “20%” of non-regulatory data is also part of the solution.
We believe that IDMP will improve the management of regulatory information, and that now is the time to build the core capabilities that will enable your organization to support it.
To learn more about Ennov RIM, please visit our website or contact us.


