The evaluation of safety signals should be a continuous process in pharmacovigilance and it is essential that Manufacturing Authorization Holders (MAH’s) have a well-defined process to capture, evaluate, communicate and take action on potential PV risks, as needed.
You have identified a signal…Now what?
Once a signal is detected, it becomes the responsibility of the MAH to determine if there is a significant risk, and if so what to do about it…
First of all, let’s make sure we are all on the same page:
The EMA defines a safety signal as “information on a new or known adverse event that may be caused by a medicine and requires further investigation. The European Medicines Agency (EMA), together with the regulatory authorities in the Member States and marketing authorization holders are responsible for detecting and managing safety signals.” They also note “The presence of a safety signal does not directly mean that a medicine has caused the reported adverse event. An illness or another medicine taken by the patient could also be the cause.”
It is these two aspects of signal detection that makes people nervous: (1) the requirement to gather information that (2) may or may not indicate that there is a safety risk with a product. Clearly there is a need to properly sort this out.
In recent years signal detection has gained a lot of interest. What is the best way to take the data we have available and apply a methodology to detect disproportionate reporting patterns? Is one statistic better than another, and if so, in every situation? What data is best for denominator comparison? How do we know if we have a “true signal” or “noise”? And the list goes on…
These are important questions that must be answered; primarily because once a signal is detected, it becomes the responsibility of the manufacturing authorization holder to determine if there is a significant risk and if so what to do about it. This is one of those things where it’s critical to, in the iconic words of Steven Covey, “Start with the end on mind”.
But Where to Start?
Before signal detection starts, it’s best to develop a clear plan for how a signal will be worked up when identified. The time to develop this plan is when cool heads prevail, not in the midst of a potential patient safety issue. Firms would do well to take the time to put together a well thought out signal detection plan, which may need to be fine-tuned for different product categories or lifecycle stages before the first signal is identified.
EMA’s Module IX – Signal management gives a good overview and launch point:
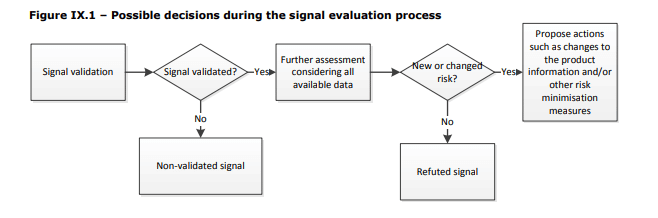
First, a signal must be evaluated to determine if it is a valid safety signal by examining the clinical relevance and the strength of evidence of the available information. In my experience it has worked well to keep this focused on the four (4) aspects of an adverse event, and look for trends:
- Case Data & Case Quality – Is the signal associated with a trend related to frequency, seasonality, temporal association, geographical location, source, etc. as compared to historical data, expectations based on product distribution or clinical data?
Is the data of good quality or are most of the cases assessed as O-unclassifiable because the information provided is insufficient or contradictory? - Patient Data – Is the signal associated with a trend related to the patient’s age, sex, race, profession, lifestyle, concomitant medical conditions, or other relevant factors?
- Product Data – Is the signal associated with a trend related to the product’s use (or misuse), lot/serial/batch, location of manufacture, labeling, distribution, or lifecycle stage?
For post-approval signal management, I usually stop here and engage my Regulatory and Quality colleagues to be aware of any product changes, ongoing activities or investigations which may be important for your signal validation. You may also want to reach out to your colleagues in Sales and Marketing to better understand the product’s activity in the field if there are any new products or competitor activity that could be driving a change in reporting, new diagnostic tests, social media buzz creating a sentinel effect, or other market factors.
Another critical piece of information to include at this point, if it is not already part of your signal detection process, is product distribution and use data – to put the volume of reports you are receiving associated with the signal into a meaningful perspective. Evaluate the reporting rates over time and by any trend that allows you to further refine your signal validation. - Event Data – Is the signal associated with a trend related to severity, frequency, time to onset, duration, progression, outcome of the event?
While this may look like a lot of work, I have found it to be more effective to objectively evaluate the points listed above rather than skipping to the most obvious or likely factor to determine if a signal is valid. Otherwise, you risk missing information and making an inaccurate conclusion.
In addition to this, one should consider if the signal is not validated, how will it be documented and followed up? Having a tool that guides you through your signal validation workflow as well as captures the data at each step can increase compliance and efficiency.
What to Do with a Validated Signal?
You have identified something that warrants further evaluation, next you must determine if this is a new risk or if this has previously been reported but changed.
It is useful to have a repository for all identified, on-going, or closed signals easily accessible to the signal management team. This allows for quick and easy assessment and decision making regarding action and communication.
Do not discard refuted signal data. Maintaining this in a searchable document management system can be very helpful for responding to NCA’s inquires or in evaluating similar signals in the future.
Time for Action
If you determine the signal is a significant safety risk, the next step is to recommend appropriate action and communication. In the same way that Quality deviations are managed, PV safety signals may require an immediate corrective action (i.e. Health Authority or Market notification, stop sale, additional studies, increased data collection, etc.) and preventative action(s). By having a process to efficiently work up signals in place, driven by a tool that makes it quick and easy to gather the information needed at each step, partnered with Regulatory, Quality, and Business Operations input, management of PV signals puts you in a strong position to mitigate risks.
To learn more about Ennov’s tool that can put this process to work for you, please visit our website or contact us.


